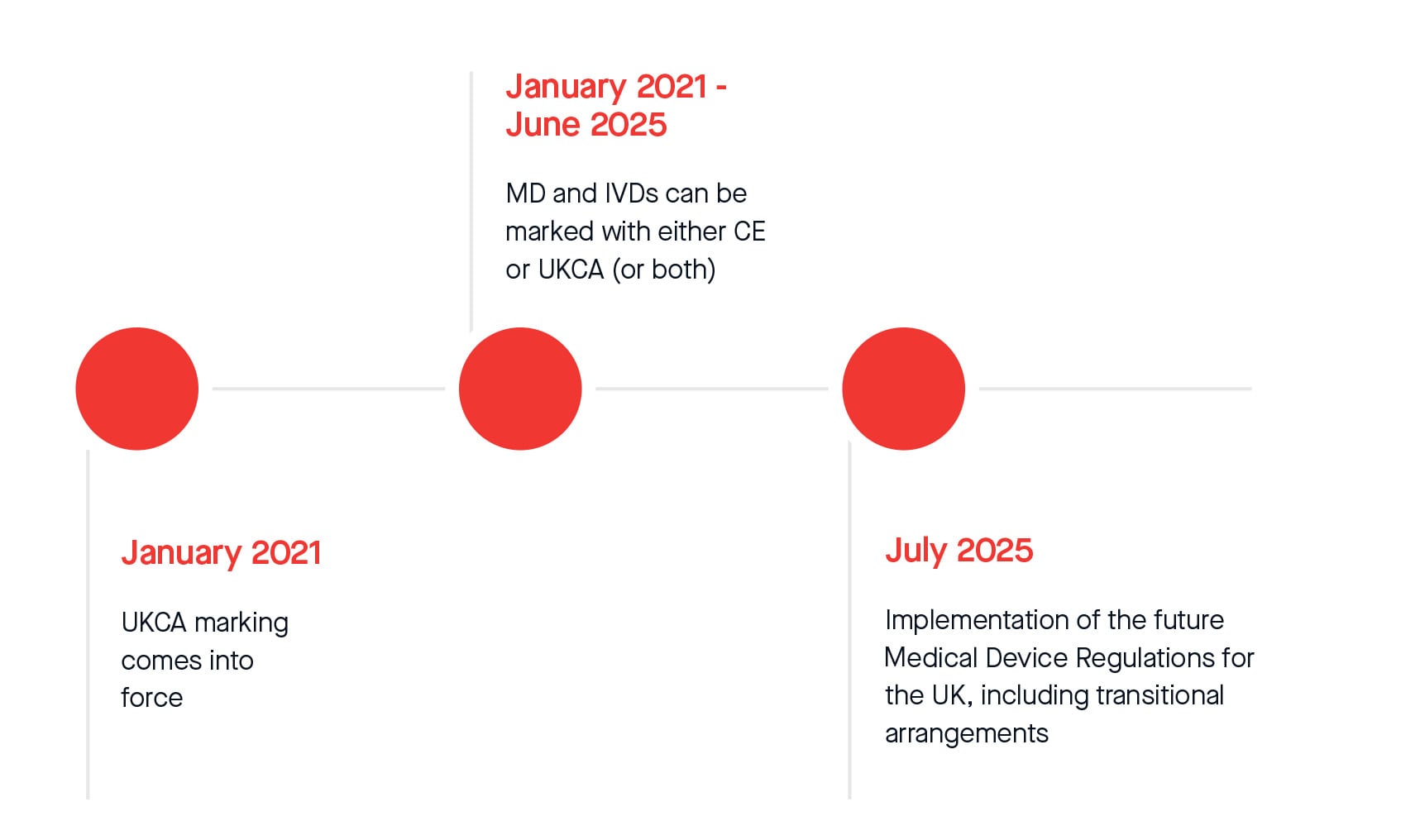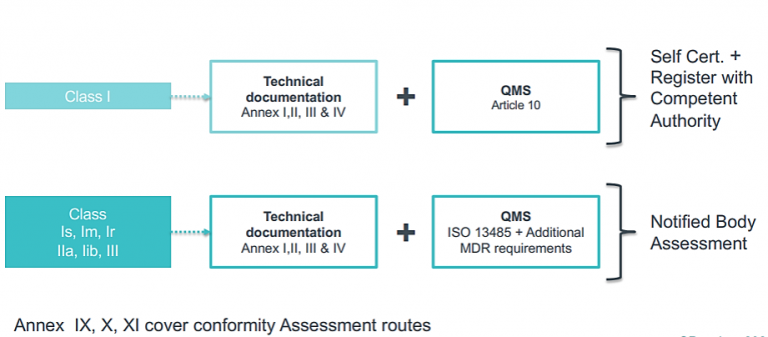
CE Marking a Medical Device under the EU MDR | Wellcome / EPSRC Centre for Interventional and Surgical Sciences - UCL – University College London

Manufacturer from Taiwan for industrial safety equipment with new CE certification - JI SAFETY Co., Ltd.

EC Certificate - Full Quality Assurance System: Directive 93/42/EEC On Medical Devices, Annex II Excluding Section 4 | PDF | Medical Device | Business